
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
Weihai Kangzhou Biotechnology Engineering Co.,Ltd
serena@whkangzhou.com
+86-0631-5678885
Model No.: Cassette
Brand: kangzhou
Place Of Origin: China
Type Of Disinfection: Far Infrared
Functional Use: Diagnostic Instruments
Inventory: Yes
Shelf Life: 3 Years
Material: Pvc
Product Quality Certification: Ce, Msds, Tuv, Ul
Medical Device Classification: Class Ii
Safety Standard: En 149 -2001+A1-2009
Use: Check if a woman is pregnant
Certificate: FDA,CE,ISO13485
Size: 2.5mm/3.0mm/4.0mm
Packaging: 1pc/foil bah , 40pcs/zipper bag or 50pcs/middle box ,
Productivity: 4000000pcs/week
Transportation: Ocean,Land,Air,Express
Place of Origin: China
Supply Ability: 4000000pcs/week
Certificate: ISO13485
HS Code: 3822190090
Port: weihai ,qingdao ,shanghai
Payment Type: L/C,T/T,D/P
Incoterm: FOB,DDU,CFR,CIF,EXW,Express Delivery
Description of desired product:
Human chorionic gonadotropin (HCG) is a glycoprotein hormone produced by the developing placenta shortly after fertilization. In normal pregnancy, hCG can be detected in both serum and urine as early as 7 to 10 days after conception. Hcg Levels continue to rise very rapidly, frequently exceeding 100 mIU/mL by the first missed menstrual period, and peaking in the 100-200 IU/mL range about 10-12 weeks into pregnancy. The appearance of hCG in both the urine and serum soon after conception, and its subsequent rapid rise in concentration during early gestational growth, make it an excellent marker for the early detection of pregnancy.
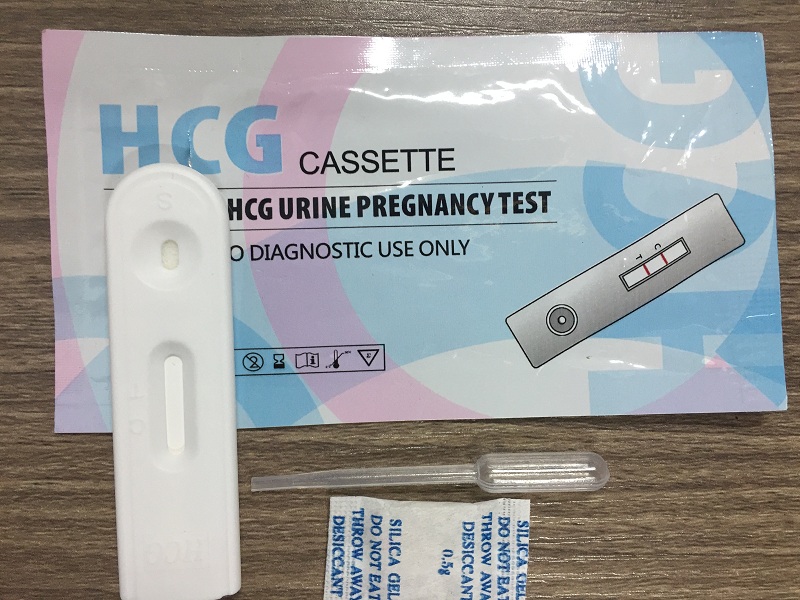
The Pregnancy One Step Rapid Test is a qualitative, solid phase, two-site sandwich immunoassay for the detection of human chorionic gonadotropin (hCG) in urine. The membrane is pre-coated with anti-hCG antibodies on the test band region and anti-mouse antibodies on the control band region. During testing, the urine sample reacts with the dye conjugate (mouse anti-hCG antibody-colloidal gold conjugate) which has been pre-coated on the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action to react with anti-hCG antibodies on the membrane and generate a red band. Presence of the red band indicates a positive result, while its absence indicates a negative result. Regardless of the presence of hCG, as the mixture continues to migrate across the membrane to the immobilized goat anti-mouse region, a red band at the control band region will always appear. The presence of this red band serves as verification for sufficient sample volume and proper flow and as a control for the reagents.
1. Remove the HCG Pregnancy test cassette from foil pouch. Use cassette t within 1 hour after removal from pouch
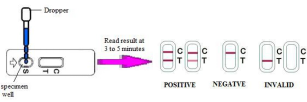
2. Draw the urine specimen using the dropper provided, and dispense 3 drops (approx. 0.1 mL) onto the sample well of the cassette (see diagram).
3. Wait for red lines to appear. The test should be read in approximately 3-5 minutes. Do not interpret results after 5 minutes.
Positive: Two distinct red lines will appear, one in the test region (T) and one in the control region (C).Positive test results are possible but not definitive for the diagnosis of pregnancy. It is strongly recommended that an additional urine specimen be obtained after 48-72 hours and tested again, or consulting your physician.
Negative: Only a single red line appears in the control region (C). No apparent red or pink line appears in the test region (T). Negative test results mean that you may not be pregnant.However, you should re-test with the first morning specimen obtained 48-72 hours later.
Invalid: Control band fails to appear which means improper testing procedures or deterioration of reagents probably occurred. In any event, repeat the test. If the problem persists, discontinue using the lot immediately and contact your local distributor.
Notes: The shade of red color in the test region (T) will vary depending on the concentration of hCG present. However, neither the quantitative value nor the rate of increase in hCG can be determined by this qualitative test.
Product Categories : HCG Pregnancy > Hcg Pregnancy Cassette


Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.